A lush planted aquarium is a wonderful thing – it’s like a little world of your own making. As exciting as the shrimp and fish can be, though, it’s important to remember that your plants need care too. As such, one of the most common questions is, ‘What is the best fertilizer?’
Here we will explore a few common fertilizer products and the situations for which they are most suitable. And, while carbon dioxide is necessary for photosynthesis and is thus extremely important for plants, this article will not explore specific CO2 -related care. This and similar non-fertilizer-specific aspects of your plant care will be left for another day.

[toc]
A recap of plant nutrition
First, as a reminder, let’s touch on the basics of plant nutrition so we know the hidden factors at play in our aquariums.
Nitrogen
From the nitrogen cycle you may already be familiar with ammonia (NH3/NH4+), nitrite (NO2-) and nitrate (NO3-). In a typical fish tank, toxic ammonia is released through the fishes’ gills and from decaying organic matter, and can be converted to nitrite by beneficial (nitrifying) bacteria, and then later converted to the nearly harmless nitrate. Plants may absorb nitrogen in the form of ammonia and nitrate, but not nitrite. Ammonia is typically not used for fertilization purposes due to its toxicity at neutral and higher pH levels. Urea can also be absorbed by plants, but it is mostly only excreted by land animals, so very little will be found in tanks.
Phosphorus
We see phosphorus in tank water in the form of phosphate (PO43-). Detritus and fish waste are both rich in phosphate.
Potassium
The potassium ion (K+) is a relatively common ion in most tap waters. Water changes and traces in food are the primary K+ sources in an unfertilized tank.
Micronutrients
Traces of iron (Fe), manganese (Mn), zinc (Zn), boron (B), copper (Cu) and molybdenum (Mo) are generally found in fish food. Micronutrients are typically chelated, meaning a chemical shell is formed around the nutrient that makes them less reactive and less likely to fall out of solution by forming insoluble salts with phosphate. The most common chelator is EDTA.
Generally abundant and/or less relevant
Calcium (as Ca2+), magnesium,(Mg2+), sulfate (SO42-) and chloride (Cl-) are all common ions in most tap waters and are rarely ever fertilized. The only real use is to add an extra bit of magnesium when your water is soft and the tank full of fast-growing plants. Nickel (Ni) and cobalt (Co) are needed for processing urea and for the growth of nitrogen-fixing bacteria. Some fertilizers do contain these elements even though they are almost entirely unnecessary to aquatic plants.
Why do we fertilize at all?
Plants need nutrients to build their own cells, and the passive influx from water changes and feeding may not always be enough. Plants’ main bulk comes from water and carbon dioxide, but of the lower-level nutrients nitrogen, phosphorus, and potassium occupy the next most critical spots. More plants means a higher demand for nutrients, and faster-growing plants need more than slower-growing ones. The exact needs of a plant differ, and certain cultivars may need a little more or less of a certain nutrient. That said, in most cases plant species from the same genus will have similar needs (e.g. all Hygrophila are very potassium-hungry).
Is fertilizer safe?
It’s possible to overdose anything, so if you add too much, it can be unsafe. Fertilizers, used more or less as intended, are very safe. The main risk ain common fertilizers is nitrate, which is generally harmless, but at very high levels can harm common stock. Very sensitive fish like growing discus (Symphysodon spp.) or bee/tiger shrimp (Caridina cf. Serrata-group) may be sensitive to moderate levels (in the single-digit ppm range). Less common culprits are the micronutrients which are technically heavy metals and are harmful in high doses. The risk of poisoning mainly lies in overfertilization and the resulting accumulation of unused fertilizer, but simply keeping up with a normal water-change schedule of >30% per week will minimize this risk.
So how do you use fertilizer?
Broadly speaking, fertilizers come in three forms: those that dissolve into the water, those that stay in the substrate, and those that make up the substrate. The decision to go with a nutrient-rich substrate or a poor one is made when setting up the tank. If using tablets and pills, it’s important to bury them properly to ensure they won’t rapidly dissolve into the water and cause sharp spikes in nutrient levels. Fertilizers intended to dissolve into the water are typically sold in liquid form, and are added to the tank in specified amounts. If you want to avoid fertilizing manually, an automated dosing pump can help reduce maintenance.
How do I know which form of fertilizer is appropriate?
These is no best or worst form and no plant will reject available nutrients and die because it comes in the wrong form. That said, rootless plants and plants floating on the surface will not be able to reach a fertilizer tablet buried deep in the substrate. Generally speaking, the water is the most accessible of the three options, but it’s important to remember that fish share it, so you can’t set the levels sky-high. Some plants only show their best colors under stress. That may be stress from too much light or from receiving limited nutrients. As such, choosing a rich substrate while limiting the water-fertilization is a way of balancing stress and nutrient availability.
Uptake through the leaves happens by diffusion. Diffusion is the property of liquids and gases to mix themselves due to the random movement of molecules. However, the water movement in the tank is naturally much faster than the speed of molecular diffusion, so flow speed is the primary deciding factor in a tank. Unless you have a dead spot of flow, the water-borne nutrient supply will be uniform throughout the tank.
Every substrate has a Cation-Exchange-Capacity (CEC). This is the slightly negative electrical charge of the soil particles that attracts positive cations which can be exchanged for one another. In practical terms, this means certain substrates can act as nutrient sponges for those nutrients that come as cations. The soil of a dirted tank and the clay and volcanic ash from aquasoils and other substrates excel in this regard, and can store certain nutrients for plants to later pick off.
Best Aquarium Plant Fertilizers Reviews
This product’s description tells us what to expect: ‘API Leaf Zone contains iron & potassium’. The Material Safety Data Sheet confirms that as well:
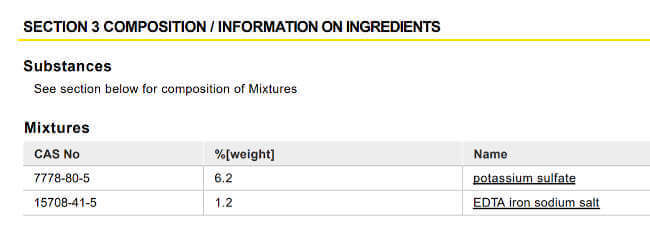
This shows us that it is just made up of potassium sulfate (K2SO4) and EDTA-chelated iron (Fe-EDTA). It does not supply other micronutrients and contains no nitrogen or phosphorus. It’s a decent choice for a typical fish tank with little plant growth under weak lighting and without additional CO2. It is suitable for a tank with abundant nitrate and phosphate in the water, but little K and micronutrients. On the downside, if you plan to grow more demanding plants with it, the lack of Mn and the other non-Fe micronutrients make it difficult to diagnose deficiencies.
API also recommends using Leaf Zone alongside API Root Tabs. According to the Material Safety Data Sheet these are made up of clay, carbon, and ‘additives’ which are unspecified trade-secret ingredients. That said, since API advertises them to be used in combination with its fertilizer, we can guess a bit. The additives will probably make up for the lack of N and P in the liquid fertilizer and may also contain the full set of micros. The clay may be there to hold it all together and add some cation exchange capacity while the carbon may absorb various substances to help prevent the additives from leaking out too quickly.
Tropica offers a description that lines up with the contents found in the analysis (.pdf-warning) given in a sponsored forum-thread. The description starts with ‘Contains iron, manganese and vital micronutrients.’ This is correct and makes it clear this is a micro fertilizer. The added note of, ‘Does not contain nitrogen or phosphor’ is also borne out in the analysis.
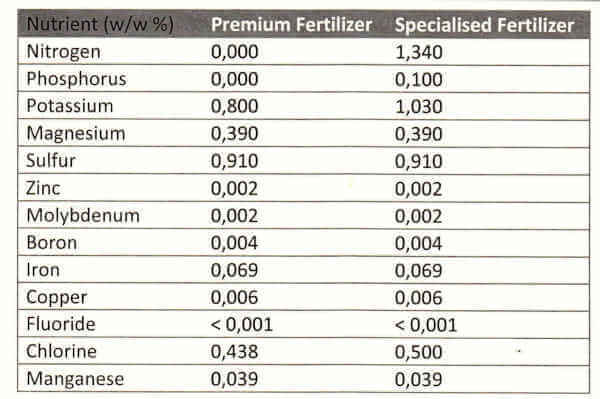
Lastly it reads ‘suitable for aquariums with few or slow-growing plants and many fish’. This is the most complex statement but makes sense and holds true when we look closer at the analysis: the fertilizer contains an ensemble of micronutrients as well as some potassium and magnesium. Potassium and micros again make sense because when they are not fertilized, the only sources are the water changes and traces in food. The addition of magnesium may not be that necessary, but it’ll help those few who have unusual tap water that is low in magnesium.
3. Tropica Specialized Nutrition
Let’s have a closer look at Tropica’s description: ‘Contains nitrogen and phosphorus for fast-growing and demanding plants,’ ‘Also contains iron, manganese, and vital micro nutrients.’ Both of these statements are clearly true according to the analysis above. Lastly, there is ‘Suitable for aquariums with many and fast-growing plants.’ This statement is a little trickier: we know N and P are added, so this is a fertilizer for tanks in which the plants demand more than the stock can produce. A high-tech planted tank with bright lights and added carbon dioxide is a typical setup that might need this. We now have to look at the ratios of nutrients. As a rule of thumb, for a non-limited approach (EI-style) the ratios of nitrate, phosphate and iron follow a rough 100:20:4 ratio, more or less.
With these calculations complete, we see that this fertilizer is a bit poor in phosphate and micros (as compared to an unlimited approach). P-limitation is a good way of bringing out the reds in plants that require stress from nutrient limitation.
4. Seachem Flourish
This Seachem Flourish formulation has the same name as the fertilizer line as a whole, making it slightly confusing. The Seachem website lists its ingredients directly. We find that Seachem Flourish is indeed comprehensive as it contains every nutrient – including those that are usually abundant – but it is essentially a micro fertilizer. Unlike the other fertilizers seen here, this is best used in combination with something else. Fortunately for us they list the substances used to make the fertilizer. Note that iron is sourced as ferrous gluconate, which is not chelated, nor do we see any mention of EDTA or DTPA, the most common chelators. The nutrients are not chelated. Flourish also contains only very little phosphate so precipitation inside the bottle is not an issue.
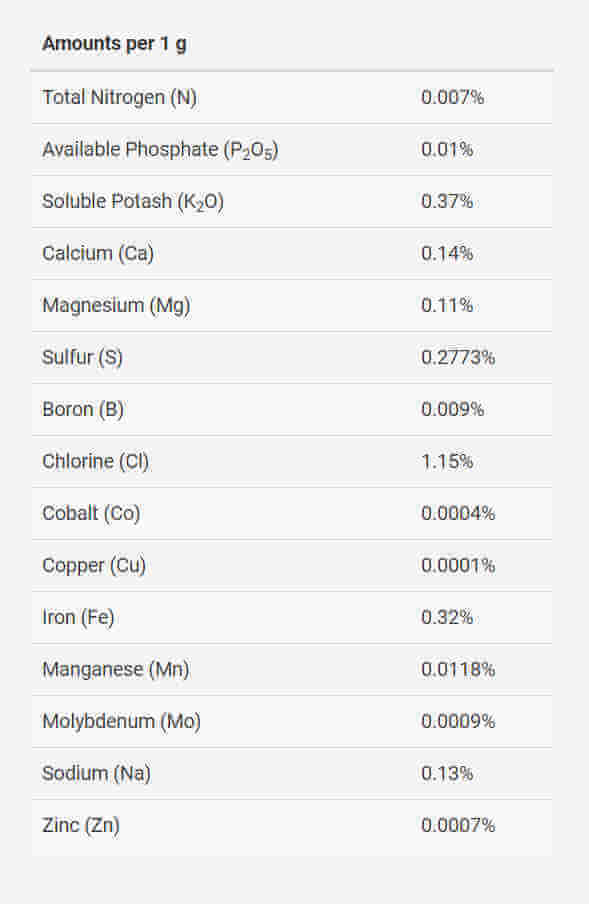
The lack of chelation means the nutrients are very strongly available for a short time, but over longer time scales may precipitate out when in contact with phosphate in the aquarium. Thus, this fertilizer is not well suited for large-interval dosing (i.e. weeks between doses) Instead, it should be dosed frequently – daily or bidaily. This is especially important if it is used to supply micronutrients to a tank with rapidly-growing plants.
5. NilocG Thrive
NilocG Thrive’s website tells us exactly what we need to know: ‘1 Pump(2ml) per 10g will add 6 ppm NO3, 1.11 ppm PO4, 4.3 ppm K, and 0.23 ppm Fe’. This is almost the exact 100:20:4 ratio needed for an Estimative Index (EI)-style unlimited approach, and this is indeed an EI-based fertilizer. It also adds extra magnesium. Thus, it’s a perfect fertilizer for a non-limited fertilization approach. But of course, it should be stated that this approach means you have to keep up on your water changes as nutrients can accumulate very easily.
There are hidden assumptions in this product, however: non-limiting means you exceed the maximum possible uptake of the plants, which in turn assumes that you have a lush tank full of more-or-less average plants under high light and additional carbon dioxide. If you, for example, wanted to grow a tank full of K-hungry Hygrophila under high light and with additional carbon dioxide, this fertilizer may offer less than the recommended dose of K required to be non-limiting.
A useful rule of thumb: If you want to do unlimited fertilization approaches in a tank without additional carbon dioxide, a quarter to a third of the corresponding dose for tanks with CO2 will be enough to be non-limiting.
Not a nutrient – the story of glutaraldehyde and ‘liquid CO2’
When discussing fertilizers, we must also mention ‘fertilizer-like’ products that aren’t technically fertilizer in the sense that they aren’t nutrients. You may have seen additives that claim to be ‘liquid CO2’. Easy Carbo by Easy Life or Flourish Excel by Seachem fall into this category.
Carbon dioxide is a gas at room temperature and pressure so to turn it into a liquid, the pressure would need to be around 50 atmospheres. As such, these products must not actually be carbon dioxide itself, but something else. What exactly are they and can they be considered useful substitutes?
So, if not a significant boost of CO2, then what good is it? Glutaraldehyde does a tiny bit of damage to every surface of living matter in contact with the tank water. Smaller organisms that naturally have a higher surface-to-volume ratio are hit harder then larger ones. Algae are of course affected much more than the larger plants or fish, a fact that’s also reflected in the lethal-concentrations documentation. What would be the best use? It operates best as an algicide to use occasionally when an algae bloom gets out of hand.
Theory: The idea of limitation
The idea of limitation is simple: plant growth is limited by the least-available nutrient. This is also known as Liebig’s law of the minimum. Ignoring light and CO2 for the moment, we can make an analogy. You can imagine each level of a nutrient as the height of one wall of a water tank, and the growth rate as the water level. The water can only rise as high as the lowest wall. This is quite a powerful idea as it means that faster growth can only be achieved by fixing all the limiting factors, not just the first one we spot.
The common example would be improving the plant growth in an unfertilized, heavily-stocked tank, more a fish tank than anything else, with low to medium lighting and no CO2 addition. There we will find that nitrates are high even if the water is changed regularly. The phosphate levels will also be high. There are few sources of potassium and micronutrients in this setup, so improving the K and micro-levels will have the greatest effect. Most of the common beginner fertilizers mainly contain micros and some also contain extra
Limiting by the available K is rarely done, but a few select species such as plants from the genus Ammannia grow well in this way. Limiting by micronutrients leads to slower growth without intense stress-colors, but can be a good way to combat algae.
Theory: ‘Deficiency’ and ‘stress-free health’ are extremes on a spectrum
So, what if a nutrient becomes even more severely limited? This is a condition referred to as ‘nutrient deficiency.’ A deficiency is the point at which the plant has trouble growing in any normal way. Thus, we can say plant health is a spectrum with deficiency at the low end. How will plants respond to severe limitation? That depends on which nutrient that is causing the deficiency.
Generally, we can classify plant nutrients into two types: mobile and immobile. Mobile nutrients are the ones the plant can redistribute at the cost of old leaves. The growing tip is the most important part of the plant and if continued survival requires the scrapping of old leaves, the plant will do so. For example, nitrogen is very mobile, and nitrogen-deficiency will appear first as lighter splotches on old leaves. This is the result of the plant literally resorbing the old leaves! Of course, mobility means you have more time to spot deficiency, but you also may have to search through the more hidden leaves to see it. Severe deficiencies in mobile nutrients can also affect younger leaves when the scrapping of old leaves is not sufficient to sustain a minimal growth rate.
Immobile nutrients are the ones the plant cannot move around. They are used more or less at the same rate they are received, meaning you find the symptoms primarily in younger leaves. Deficiencies in immobile nutrients are especially apparent in plants that grow rapidly – in rare cases they may even show up within a day or two after the plant’s nutrient supply is exhausted.
Theory: The limit of the limit – nutrient interactions
Of course, every theory has its shortcomings and Liebig’s law is no exception: it ignores interactions between nutrients, such as the fact that high-K can block the uptake of Ca. While there is the so-called Moulder’s Chart that maps out most interactions, in practical terms we only see two sets of interactions clearly. One is the antagonism between Ca, Mg, and K which all compete for uptake. In terms of ppm, the ratio of Ca:Mg:K should be kept at roughly 4:1:0.5. You may see these interactions even more clearly when you limit another nutrient at the same time. Similarly, the ppm ratio of Fe:Mn should stay between 10:1 and 4:1. That said, if you stick to a single mix with a good ratio, it’s hard for your Fe and Mn to get out of balance.
Closing remarks
Liebig’s law of the minimum and the idea of limitation and non-limitation provides a useful framework to judge fertilizer and fertilization approaches. You can find a product for nearly any approach on the market and will have to test them out to choose which is best suited for your goals. Some products may not be even be fertilizers, despite having a similar appearance. Each and every tank and combination of plants and stock will be a little different. You’ll often do better by finding your own way rather than trying to recreate your favorite picture of a planted tank.
Further Reading
JBL.de – The story of liquid CO2 fertilization
Diana Walstad – ‘Ecology Of The Planted Aquarium‘





